Introduction: Azacitidine (Aza) has been shown to prolong survival in patients with treatment-naive, higher-risk myelodysplastic syndrome (HR MDS) compared to lower-intensity, conventional treatment regimens; however, improvement in clinical outcomes are needed. Clinical data on the synergy of Aza with venetoclax (Ven)-a selective, potent BCL-2 inhibitor-in the treatment of myeloid malignancies and the unmet need in HR MDS provide a rationale to evaluate Ven in combination with Aza in treatment-naive HR MDS. Here, we report efficacy and safety data in patients with treatment-naive HR MDS treated with Ven+Aza at the recommended Phase 2 dose (RP2D) in the safety expansion part of the Phase 1b study (NCT02942290).
Methods: Adult patients were enrolled with de novo treatment-naive HR MDS defined by the Revised International Prognostic Scoring System (IPSS-R) score of >3 with an Eastern Cooperative Oncology Group performance status of ≤2, and bone marrow (BM) blasts <20% at baseline. Patients received Ven 400 mg orally daily on Days 1-14 and Aza 75 mg/m 2 intravenously or subcutaneously on Days 1-7 or on Days 1-5, 8, and 9 of each 28-day cycle (RP2D). The primary objective was to assess the complete remission (CR) rate. Key secondary objectives included determining marrow CR (mCR), overall response rate (ORR), hematologic improvement (HI), postbaseline red blood cell (RBC) and platelet transfusion independence (TI), overall survival (OS), duration of CR, time to next treatment (TTNT), transformation to acute myeloid leukemia (AML), and time to AML transformation. Responses were assessed by International Working Group 2006 criteria. Prophylactic antibiotics were mandated in Cycle 1 and recommended for Grade ≥3 neutropenia thereafter. Safety was analyzed in patients who received ≥1 dose of study treatment.
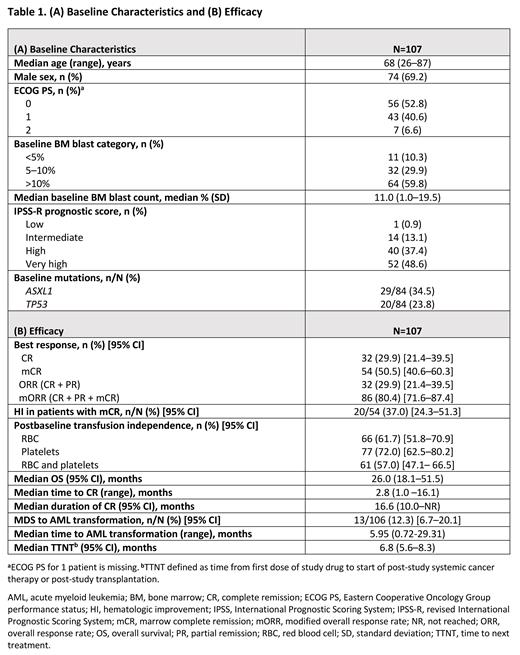
Results: At thedata cutoff of May 31, 2023, 107 patients had received Ven+Aza at the RP2D. Key baseline characteristics for all treated patients are shown in Table 1A. Median age was 68 years (range, 26-87), 64% of patients had intermediate or poor cytogenetic risk, and >89% had ≥5% BM blasts. Median number of treatment cycles before transplantation was 3.0 (range, 1.0-11.0). At the median follow-up of 31.9 months (95% CI, 30.4-42.5), CR rate was 29.9% (95% CI, 21.4-39.5), with a median CR duration of 16.6 months (95% CI, 10.0-not reached [NR], Table 1B). Median OS was 26 months (95% CI, 18.1-51.5), with an estimated 12-month OS of 71.2% (95% CI, 61.4-78.9)and 24-month OS of 51.3% (95% CI, 41.2-60.5). Median OS for CR was NR. Median TTNT including transplantation was 6.8 months (95% CI, 5.6-8.3). Sixty-two (57.9%) patients received subsequent treatment, including transplantation in 42 (39.3%) patients; the median time to transplantation was 4.8 months (range, 1.4-16.8). Among patients who received transplantation post-study, 17 were transplant ineligible at study entry. Postbaseline TI rate was 61.7% for RBC and 72.0% for platelets. Median maximal duration of TI achieved was 5.4 months (range, 1.8-50.8) and 5.4 months (range, 1.9-56.0) for RBC and platelets, respectively. Postbaseline overall hematologic improvement (HI) was achieved in 51 (49.0%) of 104 evaluable patients, and specific responses included erythroid (40/93; 43.0%), platelet (32/74; 43.2%) and neutrophil (10/57; 17.5%) indices. All patients experienced ≥1 treatment-emergent adverse event (TEAE), with 94.4% of patients reporting grade ≥3 TEAEs. Serious adverse events (SAEs) occurred in 68.2% of patients. Common TEAEs of any grade were constipation (53.3%), nausea (49.5%), neutropenia (48.6%), thrombocytopenia (44.9%), febrile neutropenia (42.1%), diarrhea (41.1%), anemia (39.3%), vomiting (36.4%), and hypokalemia (26.2%). Grade ≥3 TEAEs reported in ≥20% of patients were neutropenia (48.6%), thrombocytopenia (43.0%), febrile neutropenia (42.1%), and anemia (34.6%). Infections were reported in 57% of patients, including SAEs in 40.2% of patients. Of the 59 deaths (55.1%) reported, 23 (21.5%) were due to disease progression and 7 (6.5%) to adverse events. Within 60 days after study start, 7 deaths (6.5%) were reported.
Conclusion: Ven 400 mg for 14 days plus Aza combination was well tolerated with favorable responses of CR+PR+mCR in >80% and HI in nearly 50% of patients with treatment-naïve HR MDS. A separate Phase 3 study of this combination is ongoing to confirm survival benefit.
OffLabel Disclosure:
Garcia:Pfizer: Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy; New Wave: Research Funding; Prelude: Research Funding; Gilead: Consultancy; Bristol Myers Squibb: Consultancy; Astellas: Consultancy; AbbVie: Consultancy, Research Funding; AstraZeneca: Research Funding. Platzbecker:BeiGene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Janssen Biotech: Consultancy, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Geron: Consultancy, Research Funding; Roche: Research Funding; Celgene: Honoraria; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; AbbVie: Consultancy; Curis: Consultancy, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Merck: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Fibrogen: Research Funding. Odenike:BMS/Celgene, Novartis, Rigel, Servier, Taiho ; DSMB-Kymera therapeutics: Membership on an entity's Board of Directors or advisory committees; ABBVIE, Astrazeneca, Agios, Aprea, Astex, BMS/Celgene, CTI, Daiichi, Incyte, Janssen, Kartos, Novartis, NS-Pharma and Oncotherapy Sciences: Research Funding. Fleming:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Fong:BeiGene: Honoraria; Astellas: Honoraria; Servier: Honoraria, Speakers Bureau; Novartis: Honoraria; Pfizer: Honoraria, Speakers Bureau; Jazz: Honoraria; Otsuka: Honoraria; BMS: Honoraria; Amgen: Honoraria; AbbVie: Honoraria, Speakers Bureau. Jacoby:Gilead: Honoraria. Nowak:Pharmaxis: Research Funding; Affimed: Research Funding; Bristol Myers Squibb: Research Funding. Chyla:Abbvie: Current holder of stock options in a privately-held company; Abbvie: Current Employment. Zhou:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Ku:Genentech: Current Employment; Roche: Current holder of stock options in a privately-held company. Potluri:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Garcia-Manero:Genentech: Research Funding; AbbVie: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding.
Venetoclax Plus Azacitidine for treatment-naive high-risk MDS

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal